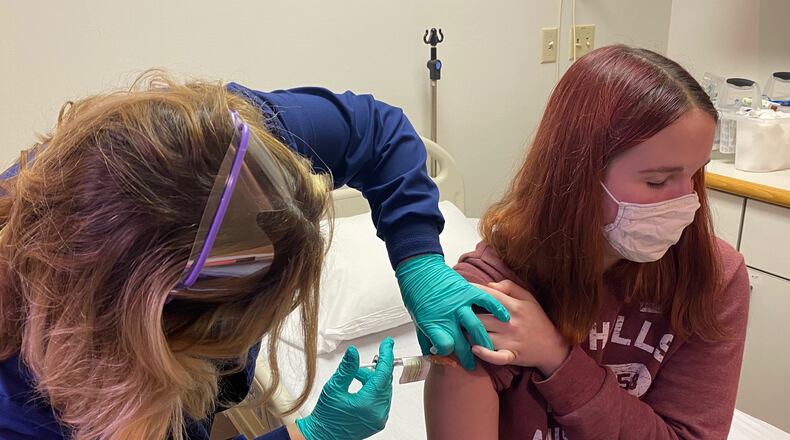
This first phase is known as “a dose-finding study,” said Dr. Robert Frenck, director of the Gamble Vaccine Research Center at Cincinnati Children’s Hospital Medical Center. No placebo will be administered in the initial group, he said.
“The study will focus on safety, side effects (if any), and immune response related to the vaccine,” Frenck said. “It will be the same type of vaccine as administered to adolescents and adults, but this trial will start with a smaller dosage.”
Cincinnati Children’s was one of the sites that began a clinical trial of the Pfizer COVID-19 vaccine with adult volunteers in May 2020. The trial was extended to 16- and 17-year-old volunteers in October 2020, and to 12- to 15-year-olds later that month, according to the hospital.
Frenck said officials expect to move down to eight children in the 2- to 4-year-old age group, then move forward with a bigger number of children.
Eventually, several hundred children 11 or younger could be enrolled at Cincinnati Children’s, he said.
Pfizer is the only authorized vaccine for children 16 and 17 years old in the United States, and the company started clinical trials in children as young as 12 last year. Moderna also started last year with its clinical trials with children, according to the vaccine maker. Less than two weeks ago, Moderna announced its study expanded to children younger than 12.
Pfizer and Moderna are the only vaccine makers at this time in clinical trials of the COVID-19 vaccine with children.
Frenck said there are two reasons to vaccinate children.
“One is the direct effect,” he said. “While the virus is less likely to cause severe disease in children, the chance is not zero.”
Of the more than 3 million children in the United States who have been infected, around 300 have died of COVID-19 and more than 12,000 have been hospitalized.
“The problem is we can’t predict which child is going to be the one to get a severe infection, so that’s why we need to immunize,” Frenck said.
About 500 families have already expressed interest in participating, according to the hospital. The later studies will include both vaccine and placebo.
Currently, more than 1,200 volunteers are participating in COVID-19 vaccine trials at Cincinnati Children’s, including more than 300 adolescents.
The clinical trial to be conducted is just at the Gamble Vaccine Research Center on the Avondale campus of Cincinnati Children’s.
HOW TO PARTICIPATE
If a parent or guardian is interested in signing up their child for later clinical COVID-19 vaccine studies with Cincinnati Children’s, they should visit: redcap.research.cchmc.org/surveys/index.php?s=FTW4CTWRCR
About the Author